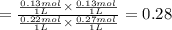Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1


Chemistry, 23.06.2019 06:30, amylumey2005
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 09:20, annapittbull12
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
Do you know the correct answer?
In an experiment, 0.35 mol of co and 0.40 mol of h2o were placed in a 1.00-l reaction vessel. at equ...
Questions in other subjects:



Social Studies, 05.02.2021 23:30

English, 05.02.2021 23:30



English, 05.02.2021 23:30

Geography, 05.02.2021 23:30


English, 05.02.2021 23:30


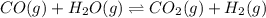
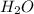 left at equilibrium = 0.40 mol - 0.13 mol = 0.27 mol
left at equilibrium = 0.40 mol - 0.13 mol = 0.27 mol![[CO]=\frac{0.22 mol}{1 L}](/tpl/images/0389/4768/0a0d1.png)
![[H_2O]=\frac{0.27 mol}{1 L}](/tpl/images/0389/4768/5bfa4.png)
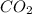 at equilibrium =
at equilibrium = ![[CO_2]=\frac{0.13 mol}{1 L}](/tpl/images/0389/4768/01e02.png)
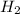 at equilibrium =
at equilibrium = ![[H_2]=\frac{0.13 mol}{1 L}](/tpl/images/0389/4768/fe16a.png)
![K=\frac{[CO_2][H_2]}{[CO][H_2]}](/tpl/images/0389/4768/437ec.png)