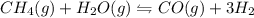Chemistry, 15.12.2019 01:31, montgomerykarloxc24x
Consider the chemical equation in equilibrium.
ch4(g) + h2o(g) < > co(g) + 3h2(g)
what will happen to the equilibrium of this reaction if the pressure is increased?
a. the equilibrium will shift to the left to favor the reverse reaction.
b. the equilibrium will shift to the right to favor the forward reaction.
c. the equilibrium will not be affected by changing the pressure.
d. the equilibrium will not be reestablished after this kind of stress.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
Do you know the correct answer?
Consider the chemical equation in equilibrium.
ch4(g) + h2o(g) < > co(g) + 3h2(g)...
ch4(g) + h2o(g) < > co(g) + 3h2(g)...
Questions in other subjects:


Mathematics, 24.03.2020 04:53


English, 24.03.2020 04:53






Mathematics, 24.03.2020 04:53