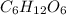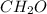Chemistry, 28.10.2019 17:31, joannamarquez0701
If a compound has a molar mass of 180 g/mol and its empirical formula is ch2o, what is its molecular formula?
ch2o
c2h4o2
c6h12o6
c12h22o11

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2


Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1
Do you know the correct answer?
If a compound has a molar mass of 180 g/mol and its empirical formula is ch2o, what is its molecular...
Questions in other subjects:




History, 11.10.2020 23:01