What are the steps needed when solving problem like this?
a compound of uranium and fluorine...

Chemistry, 14.01.2020 05:31, makaileep7449
What are the steps needed when solving problem like this?
a compound of uranium and fluorine is used to generate uranium for nuclear power plants. the gas can be decomposed to yield 2.09 parts by mass of uranium for every 1 part by mass of fluorine. if the relative mass of a uranium atom is 238 and the relative mass of a fluorine atom is 19, calculate the number of fluorine atoms that are combined with one uranium atom.
search entries or author
filter replies by unread

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01




History, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01



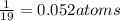
 atoms of Fluorine atoms.
atoms of Fluorine atoms.




