
Chemistry, 15.12.2019 00:31, Alexandragurule18
When an aqueous solution at room temperature is analyzed, the [h+] is found to be 2.0 × 10−3 m. what is the [oh−]?
a. 5.0 × 10−12 m
b. 2.0 × 10−11 m
c. 4.0 × 10−6 m
d. 5.0 × 10−11 m

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
Do you know the correct answer?
When an aqueous solution at room temperature is analyzed, the [h+] is found to be 2.0 × 10−3 m. what...
Questions in other subjects:




History, 02.04.2021 04:50

Mathematics, 02.04.2021 04:50



Mathematics, 02.04.2021 04:50



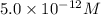
![pH=-\log [H^+]](/tpl/images/0419/0887/37e81.png)
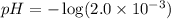
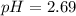
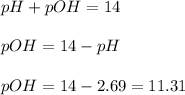
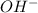 concentration.
concentration.![pOH=-\log [OH^-]](/tpl/images/0419/0887/1fac1.png)
![11.31=-\log [OH^-]](/tpl/images/0419/0887/69d8b.png)
![[OH^-]=5.0\times 10^{-12}M](/tpl/images/0419/0887/e4431.png)




