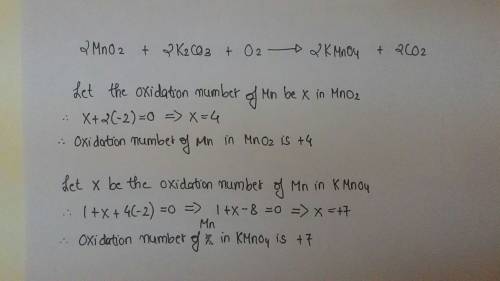Chemistry, 25.08.2019 05:20, marklynr9955
Identify the atom that increases in oxidation number in the following redox reaction. 2mno2 +2k2co3 + o2? 2kmno4 +2co2
a. o
b. mn
c. kd. c

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, qwerty8364
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1
Do you know the correct answer?
Identify the atom that increases in oxidation number in the following redox reaction. 2mno2 +2k2co3...
Questions in other subjects:

Physics, 19.01.2021 16:50

Mathematics, 19.01.2021 16:50





Mathematics, 19.01.2021 16:50