
Chemistry, 29.01.2020 00:52, waterdrop026
The reaction ab(aq)→a(g)+b(g) is second order in ab and has a rate constant of 0.0164 m −1 ⋅ s −1 at 25.0 ∘ c . a reaction vessel initially contains 250.0 ml of 0.104 m ab which is allowed to react to form the gaseous product. the product is collected over water at 25.0 ∘ c . part a how much time is required to produce 142.0 ml of the products at a barometric pressure of 707.3 mmhg . (the vapor pressure of water at this temperature is 23.8 mmhg .)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1


Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
Do you know the correct answer?
The reaction ab(aq)→a(g)+b(g) is second order in ab and has a rate constant of 0.0164 m −1 ⋅ s −1 at...
Questions in other subjects:



History, 24.01.2021 01:10

Geography, 24.01.2021 01:10







![v=k[AB]^2](/tpl/images/0479/4220/bde1b.png) . In the statement, we obtain that
. In the statement, we obtain that ![[AB]=0.104~M](/tpl/images/0479/4220/4f567.png) and, at 25 ºC,
and, at 25 ºC, 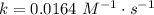 . Then:
. Then:![v=k[AB]^2\\\\ v=0.0164\cdot0.104^2\\\\ v=0.0164\cdot0.010816\\\\ v\approx0.000177=1.77\times10^{-4}~mol/s](/tpl/images/0479/4220/68b4c.png)
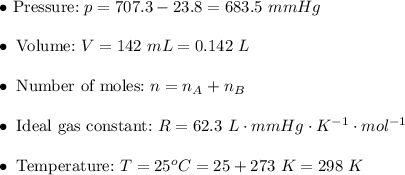

 . Hence:
. Hence: 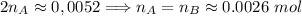 .
.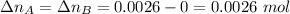 . Furthermore, since the ratio of AB to A and to B is 1:1,
. Furthermore, since the ratio of AB to A and to B is 1:1, 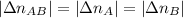 .
.![v=\dfrac{|\Delta[AB]|}{\Delta t}=\dfrac{1}{\Delta t}\cdot\dfrac{|\Delta n_{AB}|}{V}=\dfrac{1}{\Delta t}\cdot\dfrac{|\Delta n_A||}{250~mL}\\\\ 1.77\cdot10^{-4}=\dfrac{1}{\Delta t}\cdot\dfrac{0.0026~mol}{0.25~L}\\\\ \Delta t=\dfrac{0.0026}{0.25\cdot1.77\cdot10^{-4}}\\\\ \boxed{\Delta t\approx58.76~s}](/tpl/images/0479/4220/8e656.png)




