
Chemistry, 02.09.2019 02:30, adkinsryan72
15 g of hydrogen reacts with 25 g of nitrogen. how much excess reagent will be left over when the reaction stops?
5.36 g of excess
7.23 g of excess
12.12 g of excess
19.27 g of excess
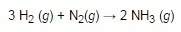

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3



Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
Do you know the correct answer?
15 g of hydrogen reacts with 25 g of nitrogen. how much excess reagent will be left over when the re...
Questions in other subjects:

Medicine, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50



Mathematics, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50


Mathematics, 07.05.2021 20:50

Mathematics, 07.05.2021 20:50






