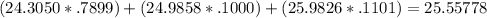Chemistry, 02.02.2020 22:56, RockieLuv7292
Magnesium has three naturally occurring isotopes: mg-24 with mass 24.3050 amu and a natural abundance of 78.99 %, mg-25 with mass 24.9858 amu and a natural abundance of 10.00 %, and mg-26 with mass 25.9826 amu and a natural abundance of 11.01 %.
calculate the atomic mass of magnesium.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 09:00, rebeccathecatt
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1

Chemistry, 23.06.2019 12:30, Vipain02
The equilibrium constant kc for the reaction 2 nocl(g) → 2 no(g) + cl2(g) is 0.453 at a certain temperature. a mixture of nocl, no, and cl2 with concentrations 1.30, 1.20, and 0.600 m, respectively, was introduced into a container at this temperature. which of the following is true? 1. no apparent reaction takes place. 2. [cl2] = 0.30 m at equilibrium. 3. nocl(g) is produced until equilibrium is reached. 4. [nocl] = [no] = [cl2] at equilibrium. 5. cl2(g) is produced until equilibrium is
Answers: 3

Chemistry, 23.06.2019 16:00, munozgiselle
Why is it important for scientists to replicate each other’s experiments? to determine if important scientific results are repeatable to the research of other scientists to determine if slight alterations in the experiment can affect the result to further their own research
Answers: 2
Do you know the correct answer?
Magnesium has three naturally occurring isotopes: mg-24 with mass 24.3050 amu and a natural abundan...
Questions in other subjects:


Mathematics, 28.04.2021 01:00


Mathematics, 28.04.2021 01:00

History, 28.04.2021 01:00


History, 28.04.2021 01:00