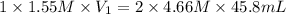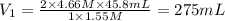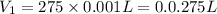Chemistry, 28.08.2019 04:30, diegobebe503
For the neutralization reaction involving hno3 and ca(oh)2, how many liters of 1.55 m hno3 are needed to react with 45.8 ml of a 4.66 m ca(oh)2 solution?
1. 0.137 l 2. 0.0343 l 3. 0.275 l 4. 1.32 l 5. 0.662 l 6. 0.330 l

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
Do you know the correct answer?
For the neutralization reaction involving hno3 and ca(oh)2, how many liters of 1.55 m hno3 are neede...
Questions in other subjects:


Geography, 20.08.2020 01:01

Mathematics, 20.08.2020 01:01






Mathematics, 20.08.2020 01:01


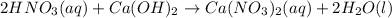

 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 
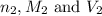 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is  .
.