
Chemistry, 27.09.2019 04:50, tayveon122
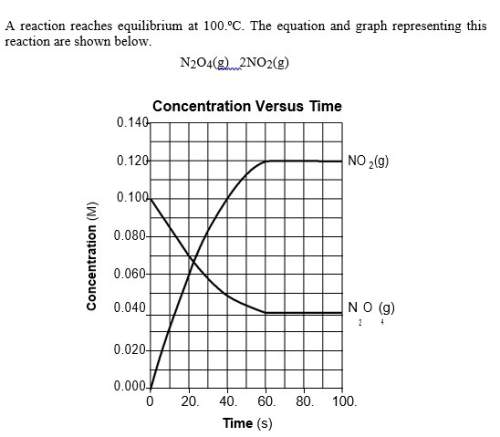
The graph shows that the reaction is at equilibrium after 60. seconds because the concentrations of both no2(g) and n2o4(g) are
(1) increasing
(2) decreasing
(3) constant
(4) zero

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1
Do you know the correct answer?
The graph shows that the reaction is at equilibrium after 60. seconds because the concentrations of...
Questions in other subjects:

Mathematics, 06.07.2019 21:30


Mathematics, 06.07.2019 21:30







Biology, 06.07.2019 21:30






