
Chemistry, 29.09.2019 13:10, Kingmoney959
According to hess’s law, if a series of intermediate reactions are combined, the enthalpy change of the overall reaction is
the difference between the enthalpies of the intermediate reactions.
the sum of the enthalpy changes of the intermediate reactions.
the product of the enthalpy changes of the intermediate reactions.
the fraction of the individual enthalpies of the intermediate reactions.

Answers: 2
Similar questions

Chemistry, 22.07.2019 01:00, dennisedemirovic20
Answers: 1

Chemistry, 28.07.2019 12:30, hamilclips1748
Answers: 2


Chemistry, 29.09.2019 08:30, whyyounodocter
Answers: 1
Do you know the correct answer?
According to hess’s law, if a series of intermediate reactions are combined, the enthalpy change of...
Questions in other subjects:

Mathematics, 04.11.2021 15:00

Mathematics, 04.11.2021 15:00

Business, 04.11.2021 15:00


Law, 04.11.2021 15:00

English, 04.11.2021 15:00

English, 04.11.2021 15:00

Mathematics, 04.11.2021 15:00

Engineering, 04.11.2021 15:00


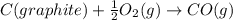
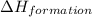
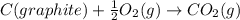

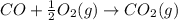
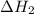
![\Delta H_{formation}=[n\times \Delta H_1]+[n\times \Delta H_2]](/tpl/images/0273/8162/6e820.png)




