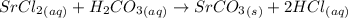Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2


Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
Do you know the correct answer?
Write a balanced net ionic equation for the following reaction: srcl2(aq) + h2co3(aq) → srco3(s) +...
Questions in other subjects:




Mathematics, 27.08.2020 01:01

Mathematics, 27.08.2020 01:01

Business, 27.08.2020 01:01

Mathematics, 27.08.2020 01:01

Mathematics, 27.08.2020 01:01

Mathematics, 27.08.2020 01:01

Physics, 27.08.2020 01:01