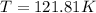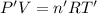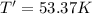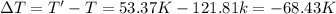Arigid, 2.50 l bottle contains 0.458 mol he. the pressure of the gas inside the bottle is 1.83 atm. if 0.713 mol ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of the gas mixture? the initial temperature of the gas is k.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 08:10, 20dyeaubn
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
Do you know the correct answer?
Arigid, 2.50 l bottle contains 0.458 mol he. the pressure of the gas inside the bottle is 1.83 atm....
Questions in other subjects:


Mathematics, 19.07.2019 15:00

Mathematics, 19.07.2019 15:00




Biology, 19.07.2019 15:00

Mathematics, 19.07.2019 15:00



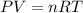 ( Ideal gas equation)
( Ideal gas equation)