
Chemistry, 07.10.2019 08:02, ilovejustinbieber42
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the solubility product constant of cu3(aso4)2.
9.1 x 10^-4 m
3.4 x 10^-2 m
3.7 x 10^-8 m
8.7 x 10^-2 m

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, carsonjohnsonn
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2


Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
Do you know the correct answer?
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the...
Questions in other subjects:



Mathematics, 19.03.2020 18:08

Mathematics, 19.03.2020 18:08








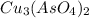 will be given by:
will be given by:

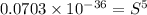
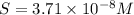
 .
.




