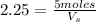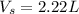Chemistry, 05.02.2020 11:46, jonathanjenkins701
How many liters of a 2.25 molar hydrobromic acid (hbr) solution would be needed to react completely with 100.0 grams of calcium metal?
ca (s) + 2hbr (aq) cabr2 (aq) + h2 (g)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, fordkenae
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1


Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Do you know the correct answer?
How many liters of a 2.25 molar hydrobromic acid (hbr) solution would be needed to react completely...
Questions in other subjects:


Mathematics, 02.10.2019 04:40


History, 02.10.2019 04:40


Advanced Placement (AP), 02.10.2019 04:40





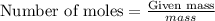 ....(1)
....(1)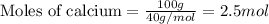
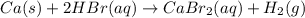
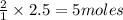 of hydrogen bromide.
of hydrogen bromide.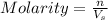
 = 5 moles
= 5 moles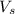 = volume of solution in liter = ?
= volume of solution in liter = ?