Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:10, kolbehoneyman
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion. what is the frequency f of oscillation?
Answers: 2


Chemistry, 23.06.2019 11:40, heyheyheyhey3
Which of the following is true for a reliable scientific source? it cites logic. it cites opinions. it cites valid data. it cites common sense.
Answers: 2

Chemistry, 23.06.2019 12:20, hayleighhurt
Describe the structure of ammonium lauryl sulfate. refer to the given diagram. your answer should include the type of bonding, the elements contained, and the size and shape of the molecule. write a short paragraph.
Answers: 3
Do you know the correct answer?
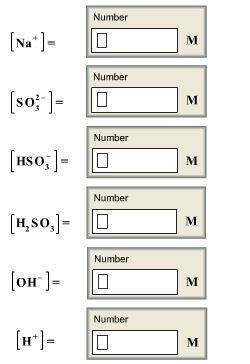
Calculate the concentrations of all species in a 1.70 m na2so3 (sodium sulfite) solution. the ioniza...
Questions in other subjects:


Biology, 26.05.2021 18:00


Mathematics, 26.05.2021 18:00





Mathematics, 26.05.2021 18:00