
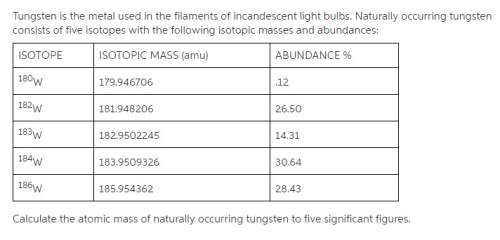
Tungsten is the metal used in the filaments of incandescent light bulbs. naturally occurring tungsten consists of five isotopes with the following isotopic masses and abundances:
calculate the atomic mass of naturally occurring tungsten to five significant figures.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1


Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
Do you know the correct answer?
Tungsten is the metal used in the filaments of incandescent light bulbs. naturally occurring tungste...
Questions in other subjects:



Mathematics, 26.08.2019 17:00

Health, 26.08.2019 17:00

Computers and Technology, 26.08.2019 17:00


History, 26.08.2019 17:00


English, 26.08.2019 17:00

Mathematics, 26.08.2019 17:00






