Me hurry
a calorimeter is a device used to measure how much heat energy is taken in or given...

Chemistry, 25.12.2019 15:31, sofiisabella10
Me hurry
a calorimeter is a device used to measure how much heat energy is taken in or given off in a reaction. suppose after five trials of a chemical reaction, 200 kj of heat was taken in on average. what conclusion can be made about the reaction? a. it is endothermic
.b. it releases heat energy.
c. it is a combustion reaction.
d. it creates more than one product.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Do you know the correct answer?
Questions in other subjects:

Advanced Placement (AP), 05.10.2021 07:50


English, 05.10.2021 07:50


Social Studies, 05.10.2021 07:50




Health, 05.10.2021 07:50

Mathematics, 05.10.2021 07:50


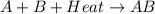
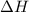 = +ve for an endothermic reaction.
= +ve for an endothermic reaction.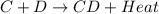 is an exothermic reaction.
is an exothermic reaction. 



