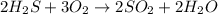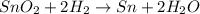Chemistry, 06.10.2019 14:30, terrieldixon
In which of the following reactions do five total molecules of reactants produce four total molecules of products? (points : 3) n2 + 3h2 2nh3 sno2 + 2h2 sn + 2h2o 2h2s + 3o2 2so2 + 2h2o 2so2 + o2 2so3

Answers: 2
Similar questions

Chemistry, 27.06.2019 22:30, shiannlacy33
Answers: 1


Biology, 25.10.2019 00:43, aarhakhanna
Answers: 2

Chemistry, 21.11.2019 14:31, jumana3
Answers: 2
Do you know the correct answer?
In which of the following reactions do five total molecules of reactants produce four total molecule...
Questions in other subjects:

Physics, 20.02.2021 06:20

Health, 20.02.2021 06:20

Mathematics, 20.02.2021 06:20

Mathematics, 20.02.2021 06:20

Arts, 20.02.2021 06:20

Mathematics, 20.02.2021 06:20

Mathematics, 20.02.2021 06:20


Mathematics, 20.02.2021 06:20

Mathematics, 20.02.2021 06:20