
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2o → oh- + sh- which of the following statements is true? na2s is a base because it ionizes to release oh-. na2s is an acid because it is a proton donor. na2s is a base because it increases the hydroxide concentration. none of these

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1


Chemistry, 23.06.2019 09:00, floressavanna15
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
Do you know the correct answer?
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2...
Questions in other subjects:


Social Studies, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00



Mathematics, 02.02.2021 22:00

English, 02.02.2021 22:00


Computers and Technology, 02.02.2021 22:00

History, 02.02.2021 22:00


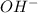 ) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution.
) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution. 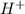 ) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.
) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.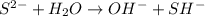
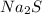 ) dissociates to give hydroxide ions.
) dissociates to give hydroxide ions. 



