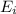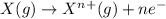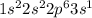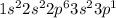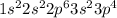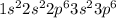Chemistry, 23.12.2019 11:31, Superman2934
According to periodic trend, which of the following most likely has the highest ionization energy? na al si ar

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 06:20, ratpizza
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 12:30, bryantjorell
An atom holds 7 electrons. use orbital notation to model the probable location of its electrons. an atom hold 22 electrons. use orbital notation to model the probable location of its electrons. an atom holds 17 electrons. use orbital notation to model the probable location of its electrons.
Answers: 1

Chemistry, 23.06.2019 12:50, vinniemccray70
What is the daughter nucleus produced a. when 217(at) undergoes alpha decay? b. when 103(mo) undergoes beta decay? c. when 188(hg) undergoes positron emission?
Answers: 1

Chemistry, 23.06.2019 13:30, leeleelynn
If a fast moving car making a loud noise approaches and moves past the person what will happen as the distance between the two increases?
Answers: 1
Do you know the correct answer?
According to periodic trend, which of the following most likely has the highest ionization energy?...
Questions in other subjects:


Chemistry, 18.10.2021 23:20




English, 18.10.2021 23:20