
Chemistry, 17.10.2019 04:00, calmicaela12s
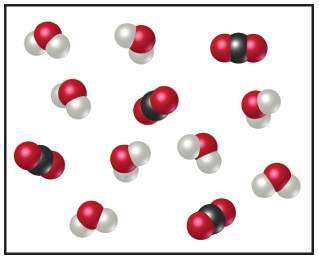
The following diagram represents the collection of co2 and h2o molecules formed by complete combustion of a hydrocarbon. (figure 1)
what is the empirical formula of the hydrocarbon?
express your answer as a chemical formula.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:50, ajaydonlee
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1
Do you know the correct answer?
The following diagram represents the collection of co2 and h2o molecules formed by complete combusti...
Questions in other subjects:

Advanced Placement (AP), 29.10.2020 21:00

Arts, 29.10.2020 21:00


Biology, 29.10.2020 21:00

Mathematics, 29.10.2020 21:00



Spanish, 29.10.2020 21:00

Business, 29.10.2020 21:00

English, 29.10.2020 21:00






