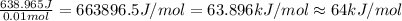Chemistry, 28.01.2020 03:31, uwunuzzles
Two solutions, initially at 24.69°c, are mixed in a coffee cup calorimeter (ccal = 105.5 j/°c). when a 200.0 ml volume of 0.100 m agno3 solution is mixed with a 100.0 ml sample of 0.100 m nacl solution, the temperature in the calorimeter rises to 25.16°c. determine the dh°rxn, in units of kj/mol agcl. assume that the density and heat capacity of the solutions is the same as that of water.
a. -78 kj/mol agcl
b. -25 kj/mol agcl
c. -64 kj/mol agcl
d. -32 kj/mol agcl
e. -59 kj/mol agcl

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 23.06.2019 07:40, Aaron5795
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 09:10, amandapill
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1

Chemistry, 23.06.2019 09:30, raymondmancilla123
The mass of a proton is approximately equal to the mass of
Answers: 1
Do you know the correct answer?
Two solutions, initially at 24.69°c, are mixed in a coffee cup calorimeter (ccal = 105.5 j/°c). when...
Questions in other subjects:




Mathematics, 27.07.2019 23:00







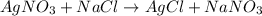
 solution ,m'=
solution ,m'=
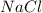 solution ,m''=
solution ,m''=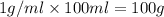
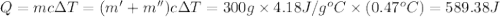
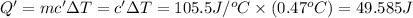
 =49.585 J+589.38 J=638.965 J
=49.585 J+589.38 J=638.965 J