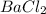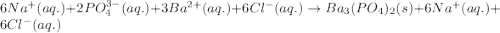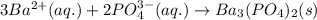Chemistry, 27.09.2019 03:30, oliwia0765
Write a balanced net ionic equation for the reactions that occur when the following aqueous solutions are mixed. include the physical states of each reactant and product. do not subscript the phases.
(a) copper(ii) sulfate, cuso4, and potassium hydroxide, koh
(b) lithium carbonate, li2co3, and aluminum nitrate, al(no3)3
(c) sodium phosphate, na3po4, and barium chloride, bacl2

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
Do you know the correct answer?
Write a balanced net ionic equation for the reactions that occur when the following aqueous solution...
Questions in other subjects:



Physics, 02.06.2021 02:30


Mathematics, 02.06.2021 02:30





Chemistry, 02.06.2021 02:30


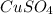 , and potassium hydroxide, KOH
, and potassium hydroxide, KOH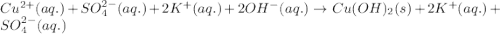
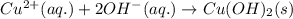
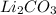 , and aluminum nitrate,
, and aluminum nitrate, 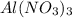
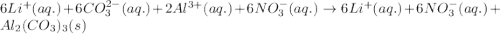
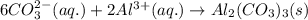
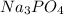 , and barium chloride,
, and barium chloride,