
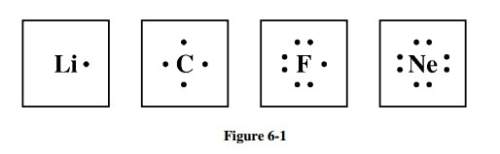
Study the electron dot diagrams for lithium, carbon, fluorine, and neon in figure 6-1. choose the statement that correctly identifies the most stable of the elements.
a. lithium is the most stable element because it has to lose only one electron to achieve a stable configuration.
b. carbon is the most stable element because it can form four bonds.
c. fluorine is the most stable element because it has to gain only one electron to achieve a stable configuration.
d. neon is the most stable element because its highest occupied energy level is filled.

Answers: 1
Other questions on the subject: Chemistry



Do you know the correct answer?
Study the electron dot diagrams for lithium, carbon, fluorine, and neon in figure 6-1. choose the st...
Questions in other subjects:








Biology, 17.04.2020 02:14

Mathematics, 17.04.2020 02:14






