
Chemistry, 04.02.2020 20:48, arianaaldaz062002
Sort each of the following events based on whether the solubility of the indicated gas will increase, decrease, or stay the same. each phrase specifies the gas involved and the change in its environment.
put the following in one of three categories.
"gas solubility increases" gas solubility decreases" " gas solubility does not change"
1. the pressure of a gas over a solvent is increase
2. the partial pressure of an anesthetic gas is increased
3. air in blood a diver descends 10 m and pressure increases by 1 atm
4. the temp is increase
5. o2 the temp of a body of water rises.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1



Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
Do you know the correct answer?
Sort each of the following events based on whether the solubility of the indicated gas will increase...
Questions in other subjects:


Mathematics, 20.09.2021 17:10



Biology, 20.09.2021 17:10

Physics, 20.09.2021 17:10





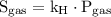
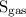 is the solubility of the gas.
is the solubility of the gas.
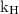 is Henry’s constant.
is Henry’s constant.
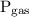 is the pressure of the gas.
is the pressure of the gas.




