
Chemistry, 17.10.2019 04:30, Jacobolobo7
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthalpy for the decomposition of 1 mole of mgo(s) into mg(s) and o2(g)?
consider this combination reaction:
what is the enthalpy for the decomposition of 1 mole of into and ?
-1204 kj/mol
602 kj/mol
1204 kj/mol
-602 kj/mol

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3
Do you know the correct answer?
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
Questions in other subjects:

Business, 22.11.2020 05:50

Social Studies, 22.11.2020 05:50





Social Studies, 22.11.2020 05:50



Mathematics, 22.11.2020 05:50


 is
is 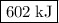 .
.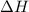 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.




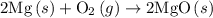
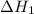 is
is 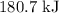 .
.
 .
. ......(2)
......(2)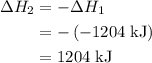
 dissociates to give two moles of
dissociates to give two moles of  and one mole of
and one mole of 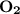 and therefore the enthalpy for the decomposition of one mole of is as follows:
and therefore the enthalpy for the decomposition of one mole of is as follows:
 .
.



