
Chemistry, 30.08.2019 22:30, Lovely3719
The acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2.
how many moles of co2 are produced when 35.0 mol c2h2 react completely? how do you get 35.0 mol out of c2h2?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Arealbot
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2


Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
Do you know the correct answer?
The acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2.
how many...
how many...
Questions in other subjects:


Mathematics, 05.02.2021 22:00

History, 05.02.2021 22:00

English, 05.02.2021 22:00


Biology, 05.02.2021 22:00




Chemistry, 05.02.2021 22:00


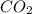 produced are, 70 moles
produced are, 70 moles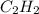 = 35 mole
= 35 mole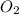 = 84 mole
= 84 mole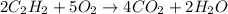
![\farc{4}{2]\times 35=70](/tpl/images/0213/2096/3d3b6.png) moles of carbon dioxide
moles of carbon dioxide



