
Chemistry, 03.10.2019 12:50, andreastyles1603
Measurements show that enthalpy of a mixture of gaseous reactants decreases by 228. kj during a certain chemical reaction, which is carried out at a constant pressure. furthermore, by carefully monitoring the volume change it is determined that -55kj of work is done on the mixture during the reaction.
calculate the change in energy of the gas mixture during the reaction.
is the reaction exothermic or endothermic?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
Do you know the correct answer?
Measurements show that enthalpy of a mixture of gaseous reactants decreases by 228. kj during a cert...
Questions in other subjects:

History, 06.02.2021 01:00

Mathematics, 06.02.2021 01:00


Arts, 06.02.2021 01:00


Mathematics, 06.02.2021 01:00


Mathematics, 06.02.2021 01:00



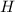 =-228 KJ
=-228 KJ 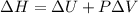
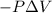
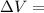 Change in volume
Change in volume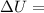 Change in energy
Change in energy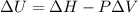

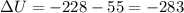 KJ
KJ



