
Amolecule of carbon dioxide (co2) contains two polar covalent bonds. why is the molecule nonpolar?
each bond in the molecule is only moderately polar covalent.
the molecule is too small to be polar.
the polar bonds are oriented in opposite directions.
the carbon atom has two unshared pairs of electrons.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Do you know the correct answer?
Amolecule of carbon dioxide (co2) contains two polar covalent bonds. why is the molecule nonpolar?...
Questions in other subjects:

Mathematics, 30.03.2021 23:40

Mathematics, 30.03.2021 23:40

Mathematics, 30.03.2021 23:40



Mathematics, 30.03.2021 23:40


English, 30.03.2021 23:40

English, 30.03.2021 23:40

Mathematics, 30.03.2021 23:40


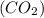 contains two polar covalent bonds. This molecule is non-polar because the polar bonds are oriented in the opposite direction.
contains two polar covalent bonds. This molecule is non-polar because the polar bonds are oriented in the opposite direction.



