
Chemistry, 24.11.2019 12:31, briannagisellegarcia
Fe2o3 + 2al -> al2o3 + 2fe
calculate the mass of iron metal (in grams) that can be prepared from 150 grams of aluminum and 250 grams of iron(iii) oxide.
!

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
Do you know the correct answer?
Fe2o3 + 2al -> al2o3 + 2fe
calculate the mass of iron metal (in grams) that can be p...
calculate the mass of iron metal (in grams) that can be p...
Questions in other subjects:





History, 01.07.2019 06:30


Mathematics, 01.07.2019 06:30

Mathematics, 01.07.2019 06:30


Mathematics, 01.07.2019 06:30


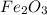 = 250 g
= 250 g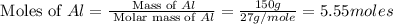
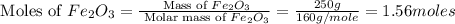
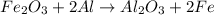
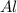 react with 1 mole of
react with 1 mole of 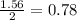 moles of
moles of 






