
Chemistry, 19.04.2021 21:20, skrillex88
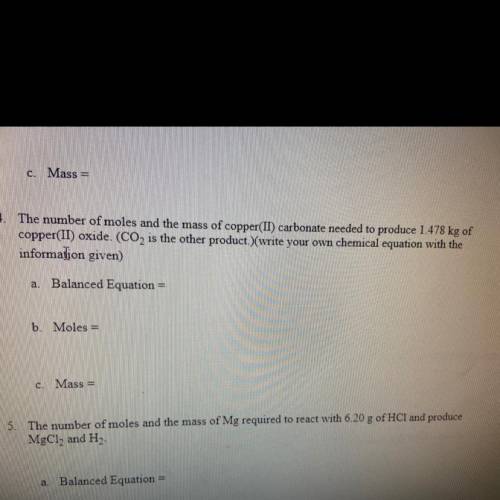
The number of moles and mass of copper carbonate needed to produce 1.478 KG of copper oxide C O two is the other products are your own chemical equation with the information given

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2


Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
Do you know the correct answer?
The number of moles and mass of copper carbonate needed to produce 1.478 KG of copper oxide C O two...
Questions in other subjects:

Social Studies, 22.08.2019 10:30



Mathematics, 22.08.2019 10:30

English, 22.08.2019 10:30

Mathematics, 22.08.2019 10:30



Mathematics, 22.08.2019 10:30

History, 22.08.2019 10:30






