
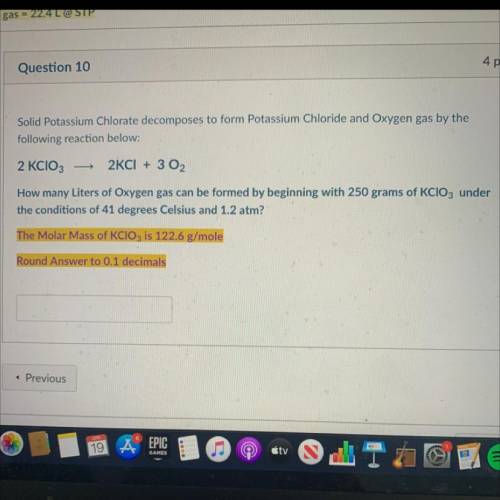
Chemistry, 19.04.2021 21:00, student679
Solid Potassium Chlorate decomposes to form Potassium Chloride and Oxygen gas by the
following reaction below:
2 KCIO3
2KCI + 3 02
How many Liters of Oxygen gas can be formed by beginning with 250 grams of KCIO3 under
the conditions of 41 degrees Celsius and 1.2 atm?
The Molar Mass of KClO3 is 122.6 g/mole
Round Answer to 0.1 decimals

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:50, kaylamount
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
Do you know the correct answer?
Solid Potassium Chlorate decomposes to form Potassium Chloride and Oxygen gas by the
following reac...
Questions in other subjects:



Mathematics, 25.10.2019 05:43

History, 25.10.2019 05:43

History, 25.10.2019 05:43

Chemistry, 25.10.2019 05:43


Mathematics, 25.10.2019 05:43

History, 25.10.2019 05:43






