
Chemistry, 19.04.2021 19:40, supasavb99
A 6.50-gram piece of aluminum reacts with an excess of oxygen. Use the balanced equation below to determine how many grams of aluminum oxide is formed during this reaction.
A.662.7 grams of Al2O3
B.24.6 grams of Al2O3
C.12.3 grams of Al2O3
D.6.1 grams of Al2O3
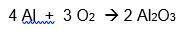

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 23:50, datboyjulio21
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
Do you know the correct answer?
A 6.50-gram piece of aluminum reacts with an excess of oxygen. Use the balanced equation below to de...
Questions in other subjects:

Biology, 23.07.2019 10:00


English, 23.07.2019 10:00












