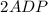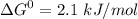Write balanced equations for all the reactions in the catabolism of glucose to two molecules of glyceraldehyde 3-phosphate (the preparatory phase of glycolysis), including the standard free-energy change for each reaction. Then write the overall or net equation for the preparatory phase of glycolysis, with the net standard free-energy change.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Queenquestion5967
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
Do you know the correct answer?
Write balanced equations for all the reactions in the catabolism of glucose to two molecules of glyc...
Questions in other subjects:

Mathematics, 27.08.2019 12:10


History, 27.08.2019 12:10



English, 27.08.2019 12:10


Biology, 27.08.2019 12:10

English, 27.08.2019 12:10

Social Studies, 27.08.2019 12:10


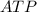 → glucose -
→ glucose - 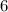 - phosphate
- phosphate 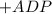 ,
, 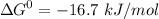
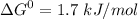
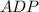 + Fructose -
+ Fructose - 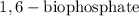 ,
, 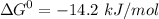
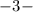 phosphate,
phosphate, 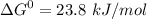
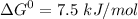
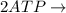 glyceraldehyde
glyceraldehyde