Chemistry, 19.04.2021 16:10, Albertrami2251
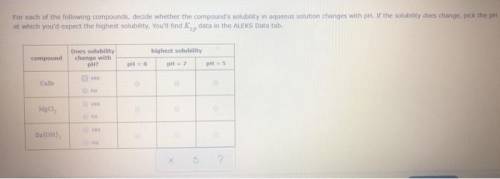
CaCO3 For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find data in the ALEKS Data tab. compound Does solubility change with pH? highest solubility pH = 5 pH = 6 pH = 7 yes no yes no yes no g

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 23.06.2019 01:50, kayleebueno
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
Do you know the correct answer?
CaCO3 For each of the following compounds, decide whether the compound's solubility in aqueous solut...
Questions in other subjects:







Mathematics, 13.01.2021 22:30


Mathematics, 13.01.2021 22:30

History, 13.01.2021 22:30