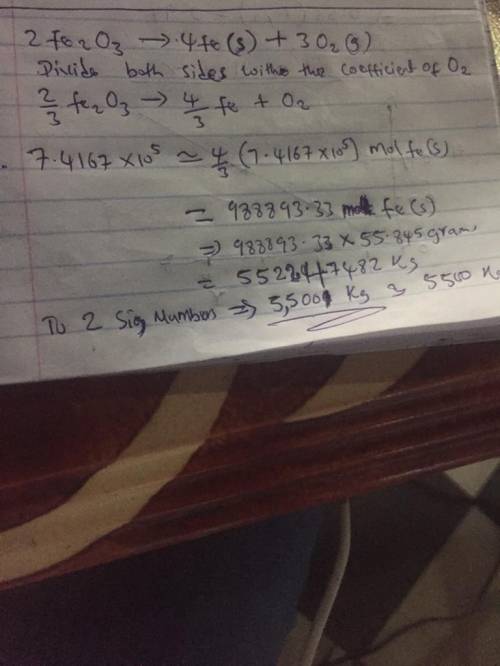Chemistry, 19.04.2021 16:10, hayleegahr
The reduction of iron(III) oxide () to pure iron during the first step of steelmaking, ()()() is driven by the high-temperature combustion of coke, a purified form of coal: ()()() Suppose at the temperature of a blast furnace the Gibbs free energies of formation of and are and , respectively. Calculate the maximum mass of pure iron that can be produced by the combustion of of coke. Round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
Do you know the correct answer?
The reduction of iron(III) oxide () to pure iron during the first step of steelmaking, ()()() is dri...
Questions in other subjects:


History, 20.09.2019 18:30



Biology, 20.09.2019 18:30


History, 20.09.2019 18:30



Social Studies, 20.09.2019 18:30