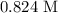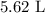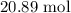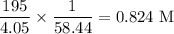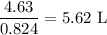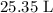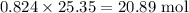Chemistry, 19.04.2021 15:50, Iamchill5998
Be sure to answer all parts. For an aqueous solution of sodium chloride (NaCl), determine the molarity of 4.05 L of a solution that contains 195 g of sodium chloride. M Determine the volume of this solution that would contain 4.63 moles of sodium chloride. L Determine the number of moles of sodium chloride in 25.35 L of this solution. mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2


Chemistry, 23.06.2019 11:20, bobthebattlebot
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1

Chemistry, 24.06.2019 02:00, jngonzo1226
Suppose you stand with one foot on ceramic flooring and one on a wool carpet, making contact over an area of 76.0 cm2 with each foot. both the ceramic and the carpet are 2.50 cm thick and are 10.0°c on their bottoms. at what rate in watts must each foot supply heat to keep the top
Answers: 2
Do you know the correct answer?
Be sure to answer all parts. For an aqueous solution of sodium chloride (NaCl), determine the molari...
Questions in other subjects:

English, 23.09.2019 16:10

Biology, 23.09.2019 16:10








Social Studies, 23.09.2019 16:10