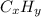Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
Do you know the correct answer?
7. A certain hydrocarbon, CxHy, is burned (reacts with O2 gas) and produces 1.955 g of CO2 for every...
Questions in other subjects:

Physics, 08.11.2020 08:30

Health, 08.11.2020 08:30

History, 08.11.2020 08:30

Mathematics, 08.11.2020 08:30

Mathematics, 08.11.2020 08:30

English, 08.11.2020 08:30

Mathematics, 08.11.2020 08:30



SAT, 08.11.2020 08:30