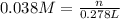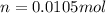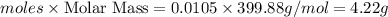Chemistry, 18.04.2021 14:00, mmcdaniels46867
Calculate the grams of solute in each of the following solution: 278 mL of 0.038 M Fe2(SO4)3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 23.06.2019 00:50, trinityine
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 09:00, sammypaige08
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
Do you know the correct answer?
Calculate the grams of solute in each of the following solution: 278 mL of 0.038 M Fe2(SO4)3...
Questions in other subjects:


English, 26.07.2019 09:30


Mathematics, 26.07.2019 09:30


Mathematics, 26.07.2019 09:30


Mathematics, 26.07.2019 09:30

Physics, 26.07.2019 09:30


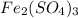
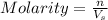
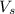 = volume of solution in L
= volume of solution in L