
Chemistry, 18.04.2021 07:50, alyssakerr17
How many moles of argon gas would be present in a 37.0 liter vessel at 45.00 °C at a pressure of 2.50 atm?
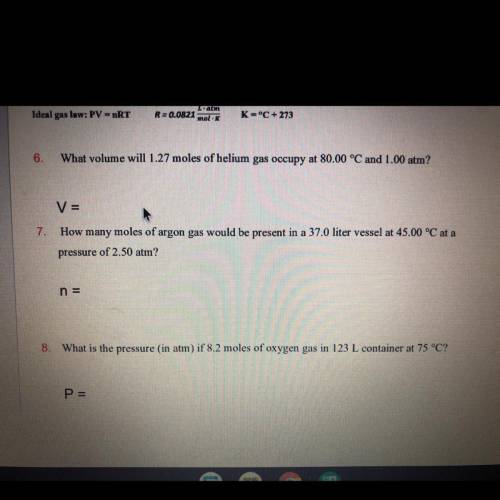

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
Do you know the correct answer?
How many moles of argon gas would be present in a 37.0 liter vessel at 45.00 °C at a pressure of 2.5...
Questions in other subjects:


Geography, 28.01.2020 20:59



Mathematics, 28.01.2020 20:59

Social Studies, 28.01.2020 20:59



English, 28.01.2020 20:59






