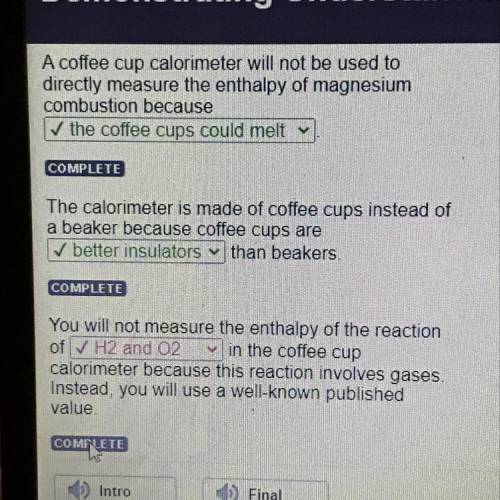
A coffee cup calorimeter will not be used to
directly measure the enthalpy of magnesium
combu...

Chemistry, 18.04.2021 05:30, GreenHerbz206
A coffee cup calorimeter will not be used to
directly measure the enthalpy of magnesium
combustion because
✓ the coffee cups could melt.
The calorimeter is made of coffee cups instead of
a beaker because coffee cups are
✓ better insulators
than beakers.
You will not measure the enthalpy of the reaction
of
✓ H2 and 02
in the coffee cup calorimeter because this reaction involves gases
Instead, you will use a well-known published
value.
These are the correct answers on edge 2021

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3


Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
Do you know the correct answer?
Questions in other subjects:





English, 30.03.2021 01:20


Mathematics, 30.03.2021 01:20



Mathematics, 30.03.2021 01:20






