
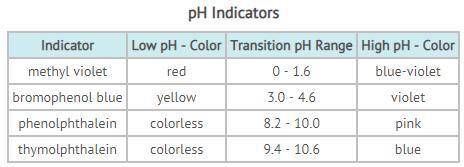
PH is an indicator of how acidic or basic a substance is. The pH scale ranges from 0 - 14, with 7 representing neutral. Acids have a pH below 7; bases, a pH greater than 7. Scientists determine the pH of a substance by using an indicator; indicators change color depending on the pH of a substance. Different indicators work at different pH ranges, as seen in the table above. The transition range of an indicator is the range of pH when the indicator changes from its low-pH color to its high-pH color. If the indicator shows a color between the two, then the pH lies somewhere in that transition range. You have been given a liquid that turns pink in phenolphthalein and blue in thymolphthalein. Tests with methyl violet and bromophenol blue resulted in a violet color. Based on your test results, determine which statements regarding the liquid are accurate. Choose ALL that apply.
A. The liquid is a base.
B. The liquid is an acid.
C. The liquid has a pH > than 7.
D. The liquid is an H⁺ donor.
E. The liquid mostly likely contains OH⁻.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 23:00, soccerplayer17
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
Do you know the correct answer?
PH is an indicator of how acidic or basic a substance is. The pH scale ranges from 0 - 14, with 7 re...
Questions in other subjects:

SAT, 14.11.2021 20:30


Computers and Technology, 14.11.2021 20:30


Social Studies, 14.11.2021 20:30

Biology, 14.11.2021 20:30









