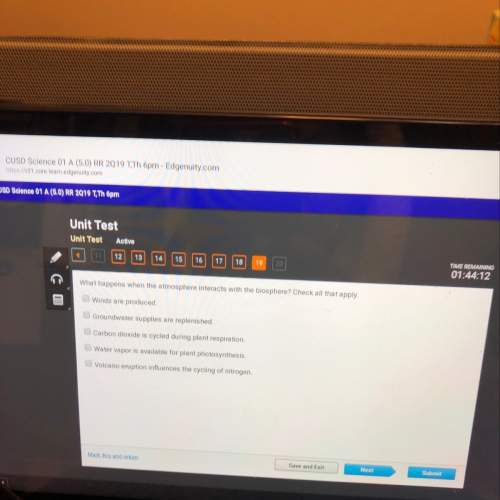
Chemistry, 16.04.2021 23:40, bmharris8262
N sub 2 +3H sub 2 rightwards arrow 2NH sub If 6 liters of hydrogen gas are used, how many liters of nitrogen gas will be needed for the above reaction at STP

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
Do you know the correct answer?
N sub 2 +3H sub 2 rightwards arrow 2NH sub If 6 liters of hydrogen gas are used, how many liters of...
Questions in other subjects:


Mathematics, 11.03.2021 23:40

Mathematics, 11.03.2021 23:40


Mathematics, 11.03.2021 23:40



Health, 11.03.2021 23:40