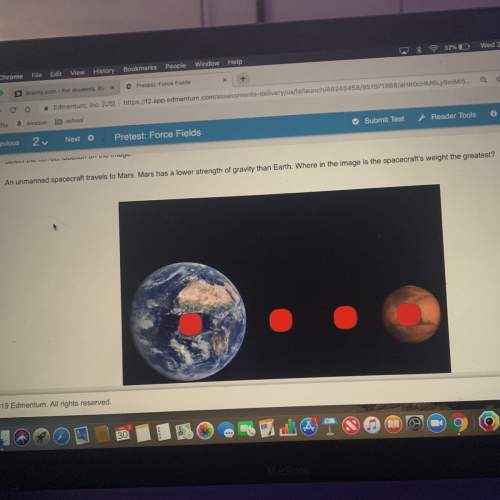
Chemistry, 16.04.2021 23:40, yairreyes01
Sodium carbonate reacts with nitric a cld according to the following equation:
Na2CO3 + 2HNO3 → 2 NaNO3 + CO 2 + H2O
If 7.50 g of Na2CO3 reacts, how many moles of CO2 are produced?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
Do you know the correct answer?
Sodium carbonate reacts with nitric a cld according to the following equation:
Na2CO3 + 2HNO3 → 2 N...
Questions in other subjects:

Physics, 07.09.2020 08:01




Computers and Technology, 07.09.2020 08:01


Mathematics, 07.09.2020 08:01


Social Studies, 07.09.2020 08:01