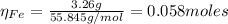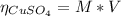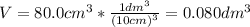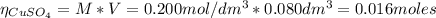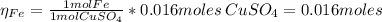Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:00, carson9373
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 19:30, TelestoisaMoon4437
What do lines in an elements line spectrum represent?
Answers: 1
Do you know the correct answer?
2.a. 3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm-3 copper(II)
sulfate solution. The...
Questions in other subjects:


Mathematics, 14.05.2021 19:50

Mathematics, 14.05.2021 19:50



Mathematics, 14.05.2021 19:50

Advanced Placement (AP), 14.05.2021 19:50

Mathematics, 14.05.2021 19:50

Mathematics, 14.05.2021 19:50

History, 14.05.2021 19:50


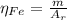
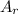 : is the standard atomic weight of iron = 55.845 g/mol
: is the standard atomic weight of iron = 55.845 g/mol