
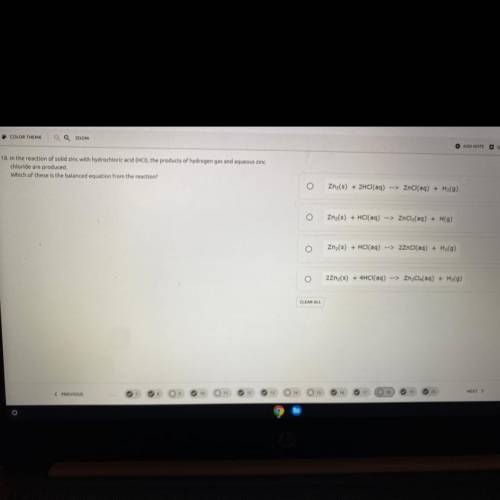
In the reaction of solid zinc with hydrochloric acid (HCI), the products of hydrogen gas and aqueous zinc
chloride are produced.
Which of these is the balanced equation from the reaction?
Zn2(s) + 2HCl(aq) --> ZnCl(aq) + H2(9)
Zn(s) + HCl(aq) --> ZnCl2(aq) + H(9)
Zn (s) + HCl(aq) --> 2ZnCl(aq) + H2(9)
22nz(s) + 4HCl(aq) --> ZnCl4(aq) + H2(9)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
Do you know the correct answer?
In the reaction of solid zinc with hydrochloric acid (HCI), the products of hydrogen gas and aqueous...
Questions in other subjects:





History, 20.03.2020 13:02

Mathematics, 20.03.2020 13:02


Health, 20.03.2020 13:02


Mathematics, 20.03.2020 13:02






