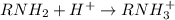Chemistry, 16.04.2021 03:20, makaylahunt
According to the Lewis model of acids and bases, amines behave as bases in water because . View Available Hint(s) According to the Lewis model of acids and bases, amines behave as bases in water because . the hydrogens will bond with hydroxide ions to make water they produce hydroxide ions they donate the hydrogens they have as hydrogen ions the unshared pair of electrons on the nitrogen can accept a proton

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
Do you know the correct answer?
According to the Lewis model of acids and bases, amines behave as bases in water because . View Avai...
Questions in other subjects:


Mathematics, 20.02.2020 02:51




Social Studies, 20.02.2020 02:51





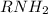 which has lone pair of electrons on nitrogen and thus is able to donate electrons to a lewis acid which is short of electrons. In other words nitrogen can accept a proton.
which has lone pair of electrons on nitrogen and thus is able to donate electrons to a lewis acid which is short of electrons. In other words nitrogen can accept a proton.