Chemistry, 16.04.2021 01:00, BreBreDoeCCx
g Consider (12.5 A) micro-grams of a radioactive isotope with a mass number of (78 B) and a half-life of (32.6 C) million years. If energy released in each decay is 32.6 keV, determine the total energy released in joules (J) in 1 (one) year. Give your answer with three significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, PSBSolarYT
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2
Do you know the correct answer?
g Consider (12.5 A) micro-grams of a radioactive isotope with a mass number of (78 B) and a half-lif...
Questions in other subjects:


Mathematics, 05.10.2019 07:30



English, 05.10.2019 07:30

Mathematics, 05.10.2019 07:30



English, 05.10.2019 07:30

Physics, 05.10.2019 07:30


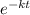
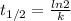
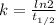
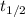 = 32.6 + 18 = 50.6 × 10⁶ years
= 32.6 + 18 = 50.6 × 10⁶ years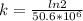 = 1.361 × 10⁻⁸ year⁻¹
= 1.361 × 10⁻⁸ year⁻¹